
Botany 321: Plant Physiology
6 December 1993

|
Susan B. Farmer Botany 321: Plant Physiology 6 December 1993
|
ABSTRACT
Anthocyanins and other secondary metabolites have been long known as being responsible for the floral coloration in various species of sessile-flowered Trillium (Trilliaceae). In this paper a biochemical basis for the three basic floral phenotypes is determined. These floral coloration patterns are based primarily on the presence or absence of certain anthocyanidins compounds. This coloration is then modIfied by the differences in concentration of several flavonol glycosides. These differences are hypothesized to be genetic action on the products of the shikimic acid pathway. Specific anthocyanins involved are cyanidin 3-arabinoside and cyanidin 3-diarabinoside. The flavonol glycosides are quercetin 3-0-glucoside, quercetin 3-0-arabinoglucoside, quercetin 3-0-arabinogalactoside, quercetin 3-0-arabinogalactosyl, 7-0-glucoside, and quercetin 3,7,0-tetraglycoside.INTRODUCTION
Since anthocyanins were first discovered in 1835, they have been known as the source for the various red and blue pigments that color plant parts such as petals, sepals, stems and fruit (Wheldale 1916). The flavones and flavonols, which are yellow pigments, can act either as pigments or as co-pigments to the anthocyanins ( Harborne 1967). The basic structure of cyanidin, an anthocyanidin, and quercetin, a flavonol, is given in Figure 1.
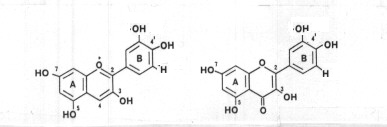 Figure 1. Structure of cyanidin (left) and quercetin (right). (from Harborne 1968). |
While phenolics and anthocyanins have been extensively studied in Trillium through two-dimensional paper chromatography (Murrell, 1969), his compounds can not be identified with any certainty as Murrell's Rf numbers do not match up with those of Harborne. However, the chromatograph that Murrell obtained for T. sessile is given in Figure 2. Murrell identifies each of the discrete spots by the color under visible and fluorescent light.
While the color variation in the floral parts of Trillium is well known as a study of the published nomenclature will quickly reveal (seemingly every color variant is given taxonomic status), little has been known of the genetic or biochemical basis of this variability until now. The photographs of Figures 3-9 illustrate the various colors of sessile-flowered trilliums.

| 
| 
|  |

| 
|  |
Figure 3-9. Photographs of Trillium pigmentation.
EXPERIMENTAL PROCEDURES
The study site was a 35 by 35 meter site northwest of the Ohio State University. In this site were approximately 18,000 individuals of T. sessile with phenotypic proportions of 93.8% red, 5.1% pink, and 1.1% yellow. Assignment of individuals to either the red or yellow classes was not difficult; however, assignment to the pink class was based upon petal color together with colorations in other parts of the plant. These features are listed in Table 1. Fifty individuals of each color were selected for analysis.Table 1. Characteristics of phenotype classes of Trillium sessile based on pigment concentrations (from Les et al., 1989).
| Petal Color | |||
|---|---|---|---|
| red | pink | yellow | |
| Petals | high | medium | none |
| Sepals | medium | none | none |
| Leaves | high | medium | none |
| Stems | high | high | none |
For each of the phenotypes, equal amounts of stem, leaf, sepal, and petal tissue were extracted, spotted onto Whatman paper and chromatographed. Extracts were also analyzed using thin-layer chromatography. The anthocyanins were purified and these purified bands were eluted and characterized using UV spectral analysis. Following treatment with trifluoroacetic acid and ethyl acetate, the anthocyanidins were resuspended and chromatographed using the FORESTAL system. The anthocyanidins were identified using the spectral and the Rf data; sugars were also identified by using their Rf values. The data from the combined spectral, color, Rf, and hydrolysis analyses were used for the final identification of the compounds.
The flavonols were analyzed only from petal tissues. While the extraction and purification process was not as stringent as that required for the anthocyanidins pigments, the identification process was basically the same for the flavonols.
RESULTS
Variations in anthocyanins were observed among the three phenotype classes. Two cyanidin (CY) anthocyanins were present: CY 3-arabinoside and CY 3-diarabinoside. The yellow trilliums had no cyanidins; the red trilliums had both CY 3-arabinoside and CY 3-diarabinoside; and the pink only had some CY 3-arabinoside. Five quercetin (Q) flavonols were identified: Q 3-0-glucoside, Q 3-0-arabinoglucoside, Q 3-0-arabinogalactoside, Q 3-0-arabinogalactosyl,7-0-glucoside, and Q 3-7-0-tetraglycoside. These were present in various concentrations among the three phenotypic classes. This data is summarized in Table 2.Table 2. Occurrence and relative concentrations of flavinoids in petals of Trillium sessile (from Les et al., 1989).
| Petal Color | |||
|---|---|---|---|
| red | pink | yellow | |
| CY 3-arabinoside | high | medium | none |
| CY 3-diarabinoside | medium | none | none |
| Q 3-0-glucoside | low | medium | low |
| Q 3-0-arabinoglucoside | high | medium | medium |
| Q 3-0-arabinogalactosyl, 7-0-glucoside | low | medium | medium |
| Q 3-7-0-tetraglycoside | none | low | low |
DISCUSSION
Having learned about the metabolic pathways that produce these types of compounds, it is interesting to study a specific example of how this relates to a specific instance in a specific plant. As was mentioned before and can easily be seen from Figures 3-9, the sessile-flowered Trillium are well known for their variation in color, and it is very interesting to see the mechanism by which this can actually occur.Based on other similarly colored flowers a genetic model of the basis for this floral polymorphism is proposed (Figure 10). Phenylalanine, a product of the shikimic acid cycle is first converted to cinnamic acid, then to flavan3,4-diol and finally to dihydroquercetin. Biochemical similarities exist between anthocyanins and flavonols as both are synthesized from dihydroflavonol. In fact, cyanidin and quercetin are both synthesized from dihydroquercetin. In Petunia hybrida the genes which regulate the conversion of dihydroquercetin into either quercetin, which is synthesized preferentially, or cyanidin have been identified.
In Petunia, quercetin synthesis is not affected by mutations that deter cyanidin synthesis indicating that anthocyanidins synthesis is not directly related to dihydroflavonol synthesis but to its conversion to cyanidin. It is evident that blocking anthocyanidins synthesis does not cause dihydroflavonol precursors to accumulate as long as flavonols can be synthesized. Therefore the prediction that the blockage of anthocyanidins synthesis would be accompanied by an increase in flavonol synthesis because of the larger pool of dihyciroflavonol precursors is borne out. Because the genetic studies that have been done have not involved heterozygous individuals, the nature of dominance cannot be determined.
Because there are no detectable anthocyanins even with the quercetin production in the yellow Trillium, a biosynthetic block exists to inhibit the conversion of dihydroquercetin to cyanidin. The increased production of tri- and tetraglycosides in the yellow plants demonstrates that the result of this block is not an excess of dihydroflavonol but rather these quercetin glycoside precursors. It is to be expected that the extra monoglycosides will provide substrate for conversion into the various polyglocosides as the sugar substitutions are made and the quantity of monoglycosides decreases. As in the Petunia, it is expected that a smaller amount of monoglycosides would be produced in the red plants due to competition for the precursors. These assumptions are borne out by the data and can be explained using a model in which a single mutation inhibits the action of the enzyme which converts dihydroquercetin to 3,4-flavan-diol or leucoanthocuanidin to cyanin.
While it is unclear whether such a mutation would have complete or incomplete dominance, the compounds in the pink plants indicate that this dominance is probably incomplete as a small amount of cyanidin monoside is produced. The pink plants also have an intermediate array of quercetins including tetraglycosides which is indicative of excess dihydroquercetin being used to produce quercetin glycosides. However, both cyanidins are present in the stem tissue of both the red and pink color types in spite of the concentrations in the petal tissue (See Table 1). Using the multiple loci involved in the genetic inheritance of color in Ipomea, it is highly likely that the actual mechanism is likely to be more complex than what is presented here.
While the primary emphasis of this paper was not on the taxonomic status of T. sessile, much has been made in the literature of the value of the chemical composition in taxonomic studies. When the chemical studies from this paper are compared with other such studies, some curious ambiguities arise. It is true that the identification of these specific compounds is not possible from Murrell's data, but the identification of cyanidin 3-diarabinoside is corroborated from Asbury's work (1972). Adams (1975) studied the flavinoids and found both quercetins and kaempferols, but this particular study found only quercetins. Unfortunately Adams' and Asbury's work cannot be compared. While they both occurred using Tennessee specimens, Asbury's work dealt with anthocyanidins pigments and Adams' with the flavinoids. Murrell's work deals with both classes of compounds, but he does not identify the compounds by class, merely by color. Whether these ambiguities are the result of geographic location or laboratory error cannot be determined with the available data. While it is obvious that the same classes of compounds are identified in each of the studies, the ambiguities in the identification of the chemical composition leads me to wonder if the study of chemical constituents warrants the taxonomic weight that many seem to give it.
CONCLUSIONS
The mechanism of floral color polymorphism of Trillium is probably quite similar to that of other species of plants as indicated by comparisons with Ipomea and Petunia. It seems that a mutation or the normal zygous state of the plant is responsible for the production of enzymes or substrate that result in the floral color within the population.It can easily be seen from published data ( Murrell 1969, Asbury 1972, Adams 1975), that these compounds are widely distributed throughout the genus. Therefore, it can be assumed with some degree of certainty, that these results (however the mechanism required to produce them) would hold true for all sessile-flowered forms of the genus.
LITERATURE REFERENCED
Back to main Trilliaceae pages
restored: 28 May 2004 sfarmer@goldsword.com